Statistical analysis of the spatial distribution and genesis of lithium in geothermal water in the Yangbajing-Gulu rift, Xizang
-
摘要:
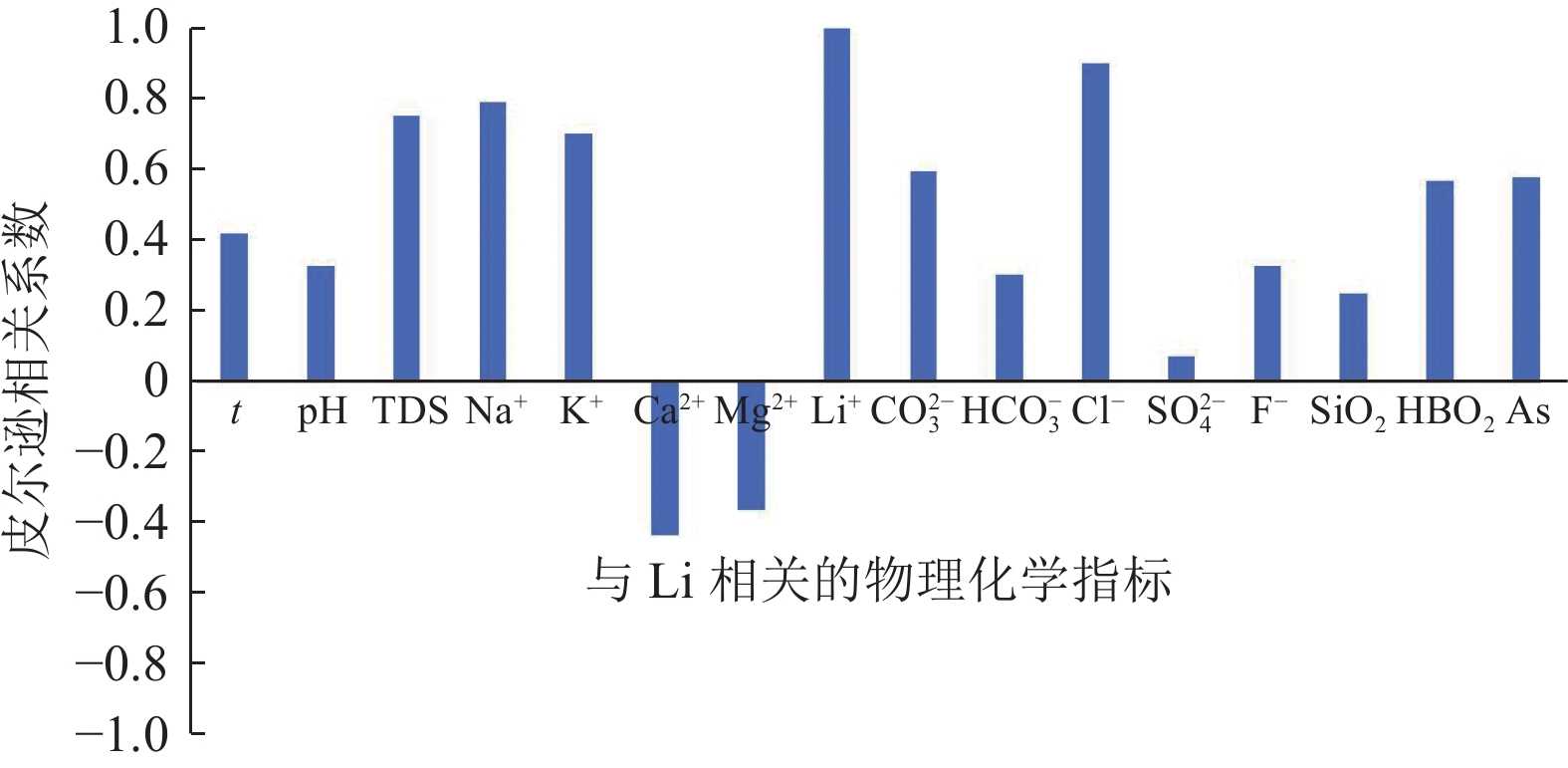
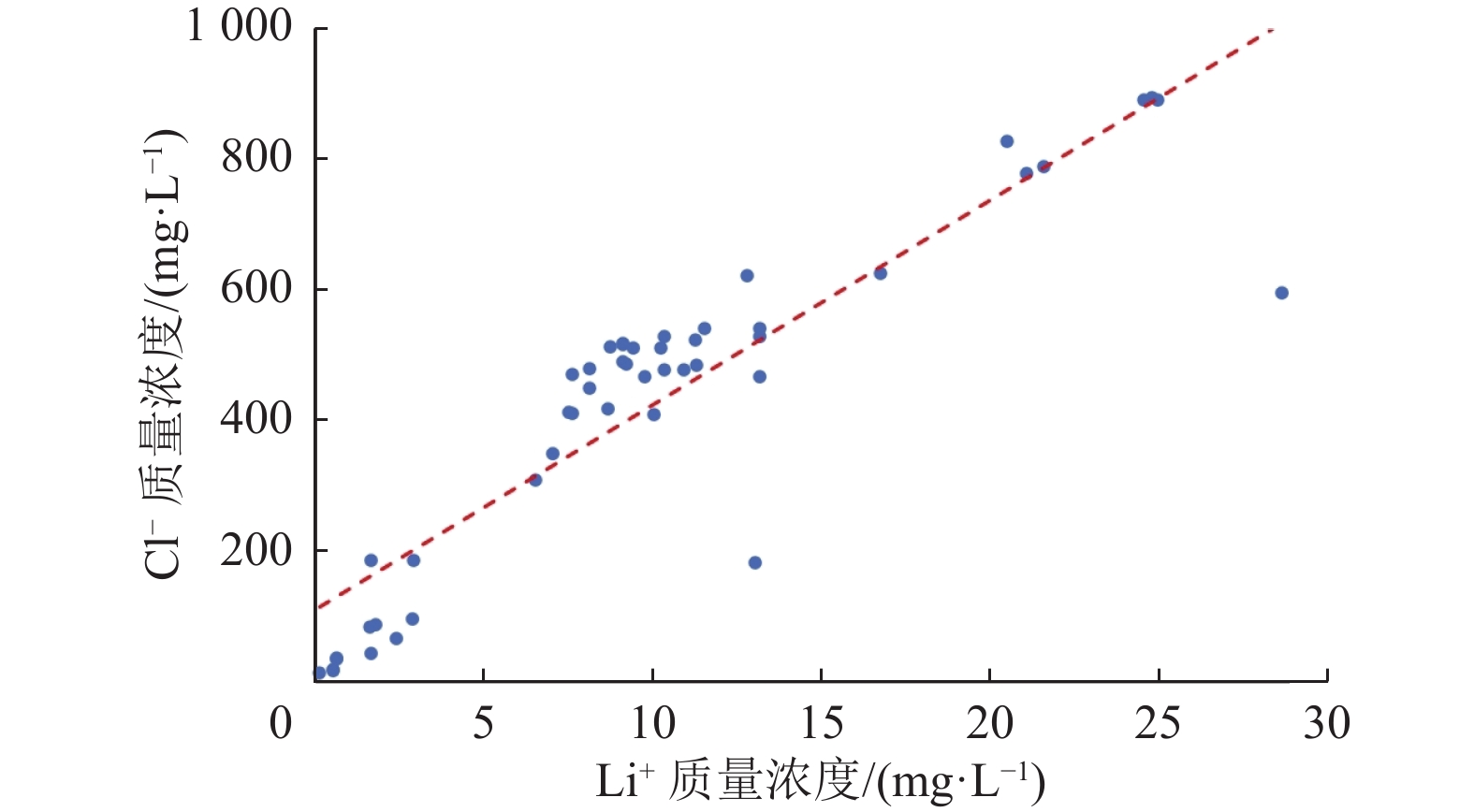
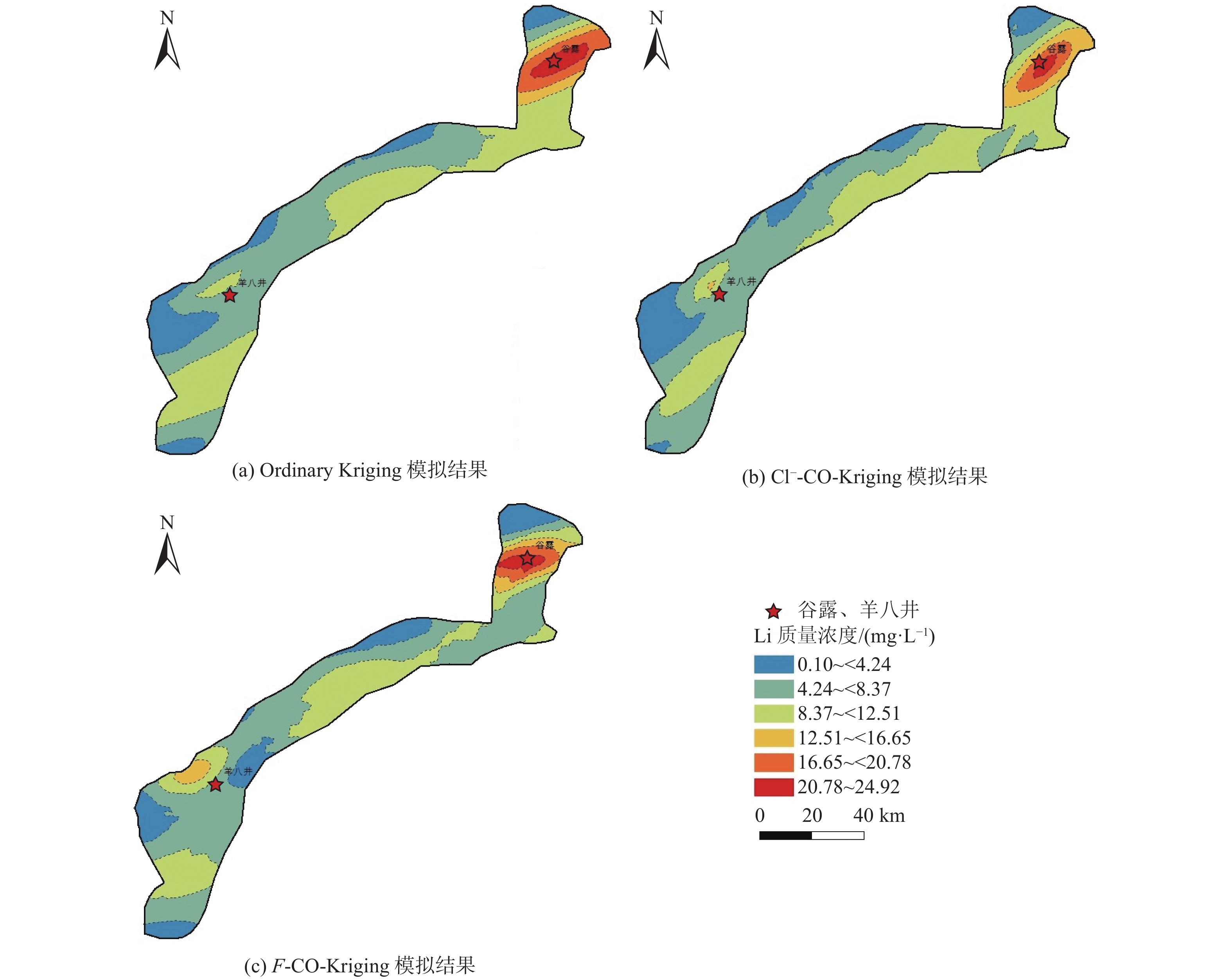
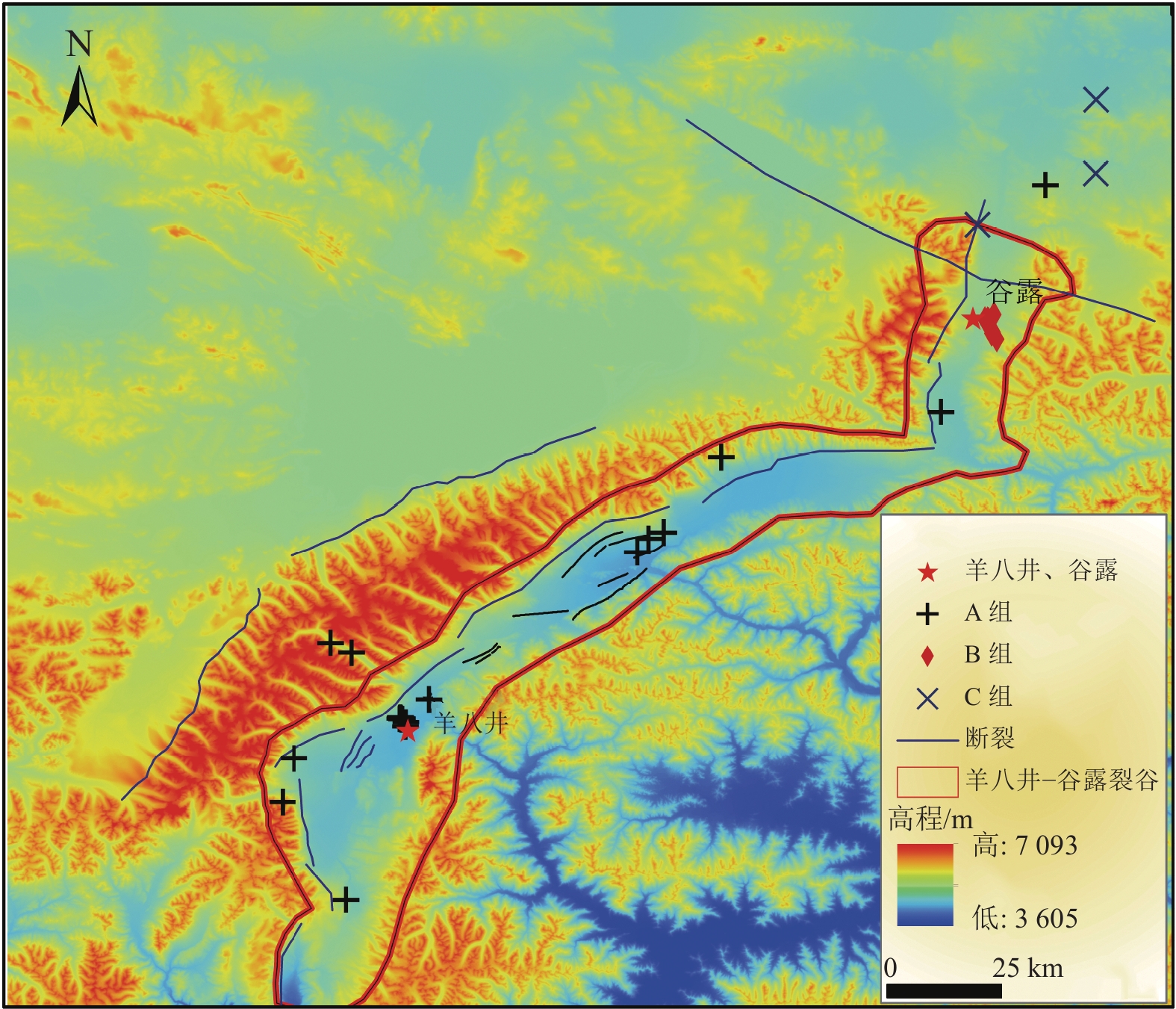
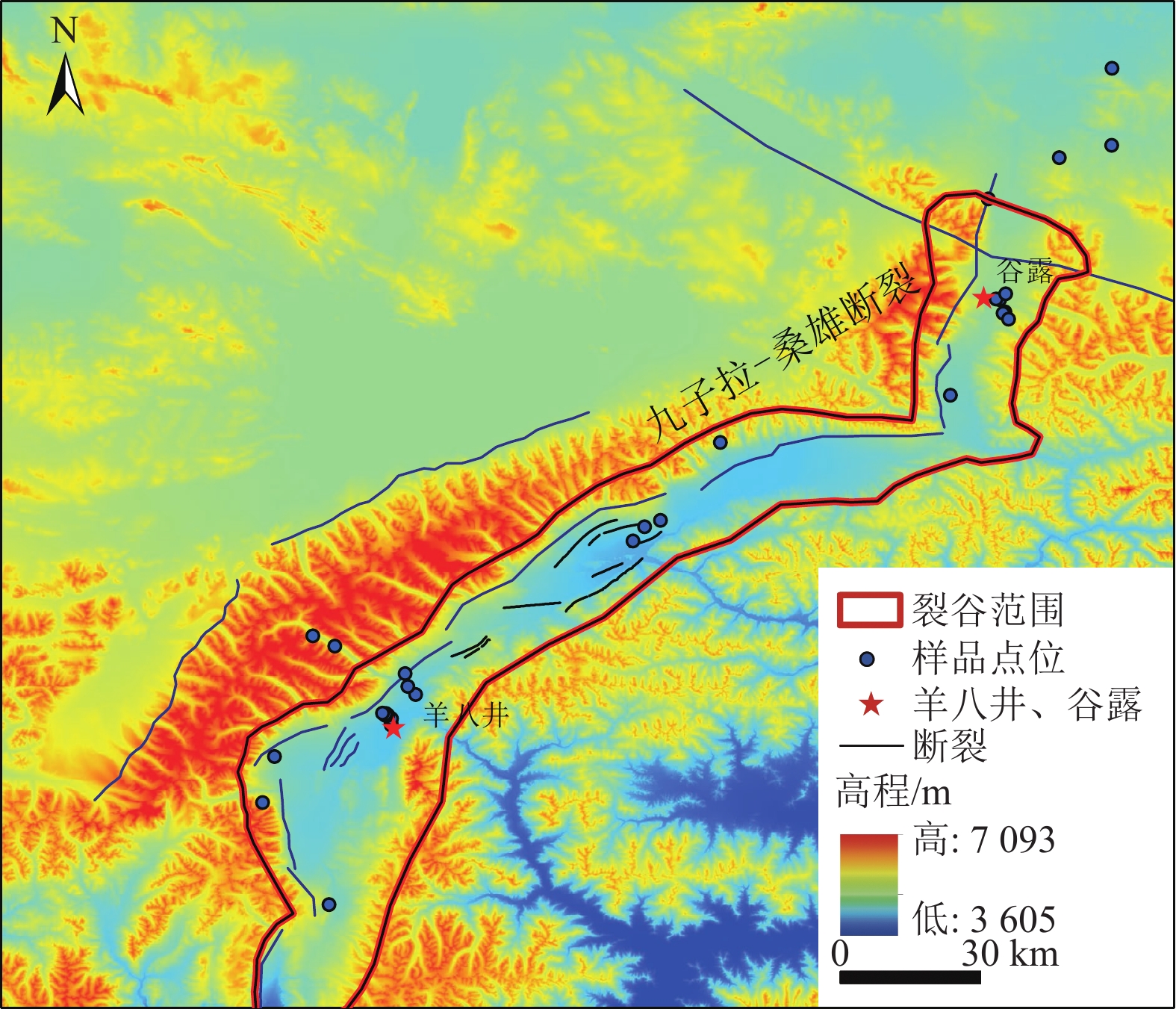
位于西藏羊八井−谷露裂谷中的地热水锂含量高于西藏温泉平均水平,但其水化学成因仍有争议,主要原因之一为该区域地热水中锂的空间分布规律不明。常见的空间规律分析方法为普通克里金法(Ordinary Kriging)和协同克里金法(CO-Kriging),但前者精度不高,后者难以获得合适的辅助变量。为此,提出2种确定辅助变量的方法:一是采用与锂相关性最强的物理化学指标Cl−浓度作为辅助变量;二是采用主成分分析综合指标F作为辅助变量。将2种辅助变量分别耦合进CO-Kriging中,形成Cl−-CO-Kriging和F-CO-Kriging方法,用以分析西藏羊八井−谷露裂谷中的地热水锂分布规律。结果表明,相比于Ordinary Kriging,F-CO-Kriging和Cl−-CO-Kriging预测精度有明显提高;其中F-CO-Kriging的EMA和ERMS平均提高30.3%,Cl−-CO-Kriging的EMA和ERMS平均提高28.5%,而且显示地热水中的锂与断裂在空间分布上具有一致性,在谷露地热区锂有明显的富集现象。进一步采用系统聚类和因子分析方法,探究影响地热水中锂空间分布的水化学成因发现,高温、高TDS、低Ca2+浓度、低Mg2+浓度、高硼浓度的碱性环境中锂浓度更高。研究成果为探讨青藏高原地热水中的高锂乃至其他稀有金属的成因和资源评价奠定基础。
Abstract:In the Yangbajing-Gulu rift, located in Xizang, the lithium concentration in geothermal water exceeds the average level of thermal springs in Xizang. However, the hydrochemical genesis of lithium in geothermal water in this rift remains controversial, and one primary reason for this is the unclear spatial distribution pattern of lithium. Common methods for analyzing spatial distribution patterns include Ordinary Kriging and CO-Kriging. Nevertheless, the former suffers low precision. For the latter, it is difficult to obtain suitable auxiliary variables. Given this, this study determined two auxiliary variables: (1) the Cl− concentration, a physicochemical parameter exhibiting the strongest correlation with lithium, and (2) comprehensive index F, as determined using principal component analysis. Integrating these two auxiliary variables separately into the CO-Kriging method formed the Cl−-CO-Kriging and F-CO-Kriging methods, which were employed to analyze the spatial distribution patterns of lithium in geothermal water in the Yangbajing-Gulu rift. The results indicate that, compared to Ordinary Kriging, both F-CO-Kriging and Cl−-CO-Kriging demonstrated significantly elevated prediction accuracy, with the former increasing EMA and ERMS by 30.3% and the latter by 28.5% on average. Furthermore, both methods revealed that lithium in geothermal water exhibits a spatial distribution consistent with faults and notable enrichment in the Yangbajing-Gulu geothermal area. This study further explored the hydrochemical genesis of the spatial distribution of lithium in geothermal water using hierarchical clustering and factor analysis. The results show that an alkaline environment characterized by high temperatures, high total dissolved solids (TDS), low Ca2+ and Mg2+ concentrations, and elevated born concentrations presents high lithium concentrations. The findings of this study will lay the groundwork for exploring the origin of high-concentration lithium and other rare metals in geothermal water on the Qinghai-Xizang Plateau and conducting relevant resource evaluation.
-
-
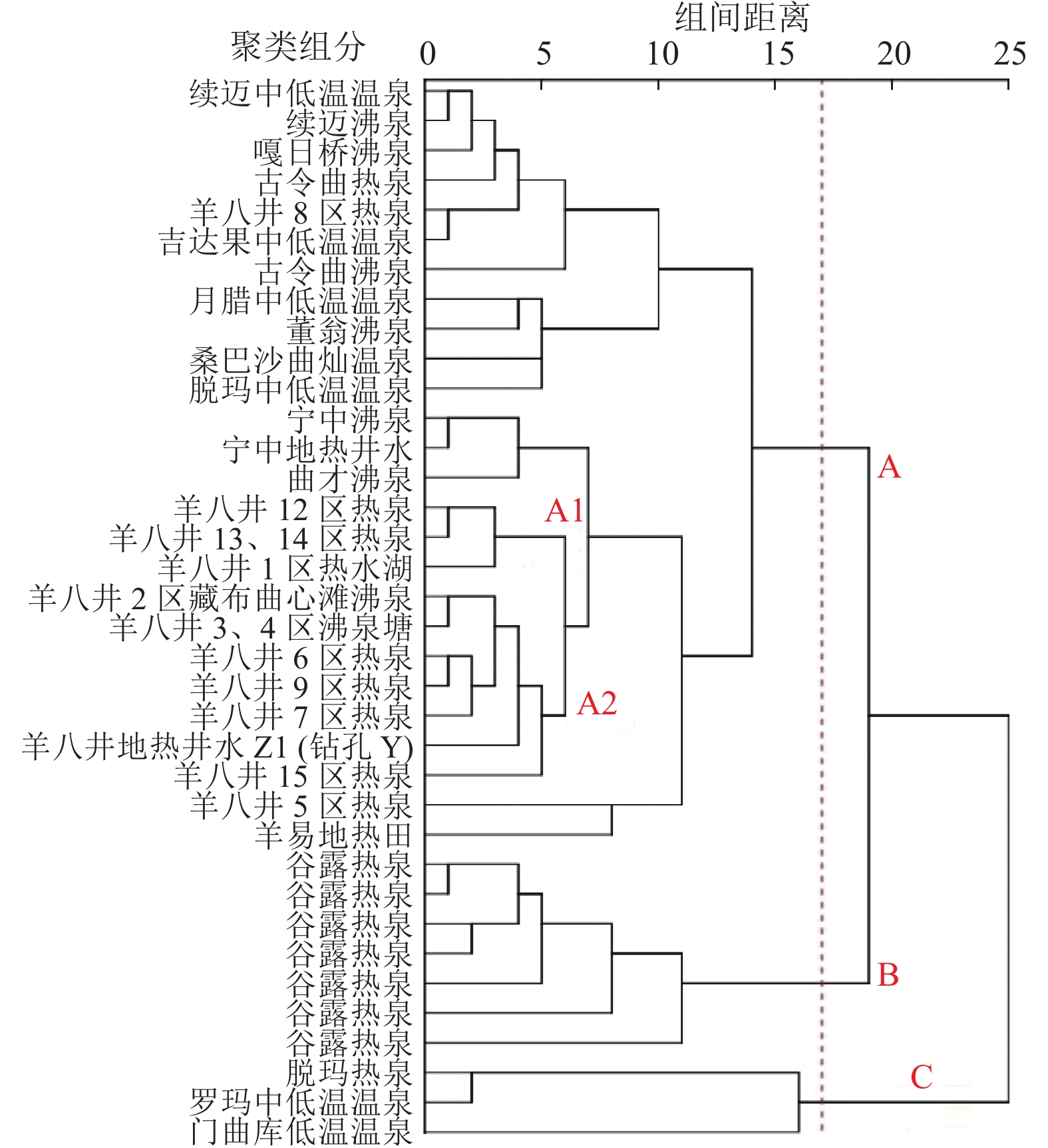
表 1 羊八井−谷露裂谷地热水水化学数据
Table 1 Hydrochemical data of geothermal water in the Yangbajing-Gulu rift
种类 温度t/℃ pH TDS/(g·L−1) 离子质量浓度/(mg·L−1) 电荷平衡/% 参考文献 Na+ K+ Ca2+ Mg2+ Li+ ${\mathrm{CO}}_3^{2-} $ ${\mathrm{HCO}}_3^- $ Cl− ${\mathrm{SO}}_4^{2-} $ F− SiO2 HBO2 As 曲才沸泉 87 8.80 2.044 532.0 131.0 0.60 2.65 10.20 60.90 496.00 512.00 192.00 7.04 203.0 132.0 1.672 −1.32 佟伟等[36] 古令曲热泉 54 8.30 0.639 195.0 10.0 4.00 0.30 0.50 0 444.00 19.00 13.50 15.40 155.0 3.5 0 0.70 古令曲沸泉 85 8.50 0.64 185.0 10.0 5.00 0.40 0.50 9.80 380.00 20.10 38.40 24.30 145.0 10.3 0 −3.06 桑巴沙曲灿温泉 39 6.75 1.54 400.0 54.0 72.00 10.40 6.50 0 819.00 311.00 41.10 2.65 56.3 175.0 0.006 2.09 羊八井1区热水湖 48 7.50 1.751 450.0 57.0 15.85 2.72 11.50 0 476.24 543.07 73.53 16.30 134.0 202.5 2.670 −3.67 羊八井2区藏布曲心滩沸泉 84 8.50 1.619 405.0 47.5 6.89 4.18 13.15 31.99 296.20 468.88 90.87 14.20 163.4 219.0 1.500 −0.86 羊八井3、4区沸泉塘 91 7.90 1.600 400.0 45.5 13.80 4.18 9.75 0 439.00 468.00 51.20 14.20 170.0 203.0 2.150 −2.79 羊八井5区热泉 87 9.20 1.877 480.0 49.0 1.38 2.30 28.60 209.07 4.64 597.13 45.44 14.70 155.0 273.4 2.650 1.70 羊八井6区热泉 80 9.00 1.656 426.0 60.5 0.20 0.15 9.10 48.80 149.00 518.00 8.42 14.90 214.0 265.0 2.534 4.33 羊八井7区热泉 83 8.80 1.686 425.0 34.0 1.72 2.72 13.15 142.23 127.89 530.55 47.93 13.60 135.0 261.2 2.670 −4.34 羊八井8区热泉 50 7.20 0.907 240.0 14.0 24.13 4.18 2.90 0 339.80 187.12 45.44 6.50 86.0 121.5 0.216 2.54 羊八井9区热泉 87 8.90 1.823 467.5 58.5 1.72 5.23 13.15 74.59 185.84 542.86 66.09 14.90 171.5 303.7 2.500 2.63 羊八井12区热泉 67 6.60 1.329 310.0 34.0 25.85 2.30 7.00 0 376.37 350.31 46.25 9.70 194.3 157.1 0.920 −1.97 羊八井13、14区热泉 58 7.20 1.399 365.0 39.0 9.60 1.05 10.00 0 369.36 410.05 55.91 11.50 158.3 148.0 1.620 −1.33 羊八井15区热泉 85 7.70 1.891 467.5 58.5 10.30 2.08 11.25 0 438.47 525.70 41.12 6.20 247.6 298.0 2.250 1.99 羊八井地热井水Z1(钻孔Y) 84 8.60 1.790 445.0 53.5 5.15 1.03 12.75 62.83 178.91 623.65 53.46 6.50 124.8 303.0 4.000 −2.49 羊易地热田 87 9.70 1.708 450.0 30.0 1.00 0.10 13.00 200.00 357.00 183.00 217.00 23.50 258.0 150.0 1.820 −2.59 门曲库低温温泉 29 8.00 0.670 150.0 8.2 48.00 28.10 0.10 0 482.00 14.30 132.00 2.06 31.1 11.0 0 1.44 脱玛热泉 51 6.80 2.220 570.0 72.5 117.00 18.70 0.60 0 1020.00 38.20 798.00 6.65 78.9 15.0 0 −0.92 谷露热泉 86 9.14 3.87495 1138.2 130.36 0 0 24.52 261.65 969.56 893.42 235.22 15.20 146.70 59.23 1.25 0.76 张萌等[35] 谷露热泉 94 8.85 4.06234 1098.0 138.28 0 0 24.77 133.73 1235.59 896.96 219.35 15.27 131.74 168.12 1.37 −0.48 谷露热泉 83 8.54 4.40290 1253.6 134.72 0 0 24.92 81.40 1318.36 892.07 389.70 14.50 135.72 156.79 1.47 2.66 谷露热泉 61 7.30 4.35387 1165.5 115.06 12.83 3.89 20.45 0 1433.05 829.66 370.25 11.80 240.17 149.83 1.40 2.05 谷露热泉 84 8.15 3.93883 998.0 121.22 4.81 1.95 21.03 0 1513.46 779.97 192.22 12.40 131.61 161.15 1.29 −1.51 谷露热泉 68 7.64 3.77993 953.4 137.20 3.21 0.97 21.55 0 1377.48 790.60 179.53 12.60 156.98 145.47 1.23 −0.99 谷露热泉 44 7.01 3.54220 831.3 105.80 48.12 13.62 16.71 0 1460.25 627.52 125.31 7.20 179.37 125.44 0.58 0.20 续迈中低温温泉 78 8.22 0.68951 149.59 9.76 8.02 0.97 1.77 10.49 96.98 89.34 144.16 8.85 120.26 47.91 0.19 −2.83 刘昭等[38] 续迈沸泉 86 8.20 0.70492 152.95 9.84 8.02 0.97 1.58 9.30 96.96 85.09 153.59 9.40 129.87 46.17 0.28 −2.44 吉达果中低温温泉 64 7.29 1.18173 228.68 14.93 40.10 4.86 2.84 0 472.95 97.85 138.01 6.20 116.64 58.36 0.29 −2.12 刘昭等[38] 嘎日桥沸泉 80 8.17 1.26264 273.59 21.88 16.04 1.95 2.37 0 679.87 67.36 38.81 9.30 105.67 45.30 0.27 −2.07 宁中沸泉 88 8.27 2.61245 637.25 116.28 30.48 7.78 10.88 58.14 621.94 479.32 362.44 7.45 128.47 148.08 1.95 1.23 宁中地热井水 80 7.70 2.65201 620.15 109.66 32.08 10.70 11.26 0 747.27 486.42 350.23 7.00 128.48 144.60 2.14 0.39 月腊中低温温泉 69 7.03 1.47051 239.59 36.54 70.58 14.59 1.63 0 856.05 44.67 59.59 2.36 116.49 27.00 0 −0.99 董翁沸泉 86 7.31 1.46048 279.56 60.00 69.95 7.81 8.65 0 454.04 419.59 13.46 3.20 67.59 74.91 0.79 −1.64 脱玛中低温温泉 55 7.48 2.49132 549.45 68.56 60.95 11.67 1.62 0 1531.46 187.19 5.25 6.20 62.08 24.39 0 −1.51 罗玛中低温温泉 49 7.17 2.56408 541.48 68.40 117.09 10.21 0.61 0 997.93 36.16 678.33 5.30 86.86 20.91 0 0.47 羊八井地热井水Z2 8.86 417 48.4 1.89 0.10 10.3 479 32.8 13.2 205 赵平等[37] 羊八井地热井水Z3 8.82 328 38.5 3.99 0.10 7.5 415 35.6 13.7 206 羊八井地热井水Z4 8.88 338 44.2 3.64 <0.01 7.6 472 40.8 14.7 224 羊八井地热井水Z5 8.89 337 39.6 4.40 0.10 8.1 481 39.7 14.9 214 羊八井地热井水Z6 8.21 316 35.7 3.57 <0.05 7.6 412 33.8 13.2 216 羊八井地热井水Z7 8.17 332 41.7 3.89 <0.05 8.1 451 34.6 13.2 217 羊八井地热井水Z8 8.80 377 44.8 3.70 <0.05 9.1 491 38.1 14.2 238 羊八井地热井水Z9 8.93 387 45.8 3.19 0.10 9.4 513 38.4 14.3 248 羊八井地热井水Z10 8.49 408 47.3 3.56 <0.05 9.2 489 37.7 14.6 241 羊八井地热井水Z11 8.90 421 55.6 2.82 <0.05 10.3 531 39.6 14.0 260 羊八井地热井水Z12 8.94 383 48.4 3.97 <0.05 9.1 519 39.9 15.1 247 羊八井地热井水Z13 8.92 370 47.5 2.84 <0.01 8.7 514 43.2 14.4 256 注:电荷平衡[39]=$[(\displaystyle\sum {阳离子} - \left| {\displaystyle\sum {阴离子} } \right|) \div (\displaystyle\sum {阳离子} + \left| {\displaystyle\sum {阴离子} } \right|)] \times 100$%。 表 2 各物理化学指标之间皮尔逊相关系数
Table 2 Pearson correlation coefficients between various physicochemical indices
指标 t pH TDS Na+ K+ Ca2+ Mg2+ Li+ ${\mathrm{CO}}_3^{2-} $ ${\mathrm{HCO}}_3^{-} $ Cl− ${\mathrm{SO}}_4^{2-} $ F− SiO2 HBO2 As t 1 0.667 0.139 0.170 0.219 −0.574 −0.617 0.418 0.483 −0.263 0.382 −0.125 0.434 0.431 0.390 0.507 pH 1 0.057 0.028 −0.004 −0.711 −0.516 0.328 0.752 −0.332 0.313 −0.263 0.673 0.521 0.330 0.487 TDS 1 0.985 0.918 −0.064 −0.093 0.754 0.265 0.798 0.788 0.454 0.109 0.201 0.170 0.208 Na+ 1 0.909 −0.077 −0.125 0.790 0.342 0.743 0.760 0.473 0.114 0.028 0.197 0.239 K+ 1 −0.002 −0.038 0.703 0.235 0.688 0.735 0.465 −0.046 0.008 0.198 0.273 Ca2+ 1 0.757 −0.438 −0.408 0.302 −0.491 0.539 −0.677 −0.620 −0.483 −0.539 Mg2+ 1 −0.367 −0.340 0.236 −0.391 0.331 −0.651 −0.593 −0.377 −0.407 Li+ 1 0.598 0.301 0.900 0.072 0.328 0.247 0.568 0.580 ${\mathrm{CO}}_3^{2-} $ 1 −0.155 0.410 0.020 0.482 0.271 0.302 0.423 ${\mathrm{HCO}}_3^{-} $ 1 0.354 0.421 −0.144 −0.128 −0.291 −0.311 Cl− 1 −0.029 0.285 0.383 0.616 0.628 ${\mathrm{SO}}_4^{2-} $ 1 −0.204 −0.285 −0.284 −0.162 F− 1 0.592 0.229 0.319 SiO2 1 0.490 0.492 HBO2 1 0.876 As 1 表 3 主成分特征值和累计贡献率
Table 3 Eigenvalues and cumulative contribution rates of principal components
成分 初始特征值 提取载荷平方和 总计 方差百分比/% 累积/% 总计 方差百分比/% 累积/% F1 5.526 42.508 42.508 5.526 42.508 42.508 F2 3.704 28.494 71.003 3.704 28.494 71.003 F3 1.337 10.285 81.287 1.337 10.285 81.287 F4 0.868 6.674 87.962 F5 0.566 4.352 92.313 F6 0.307 2.363 94.677 F7 0.294 2.261 96.937 F8 0.130 0.998 97.935 F9 0.112 0.864 98.799 F10 0.080 0.614 99.413 F11 0.042 0.327 99.740 F12 0.029 0.222 99.961 F13 0.005 0.039 100.000 表 4 Cl−-CO-Kriging、F-CO-Kriging与Ordinary Kriging精度对比
Table 4 Accuracy comparison between the Cl−-CO-Kriging, F-CO-Kriging, and Ordinary Kriging methods
插值方法 EMA ERMS 数值 精度提升/% 数值 精度提升/% Ordinary Kriging 1.79 0 2.72 0 Cl−-CO-Kriging 1.25 30.2 1.99 26.8 F-CO-Kriging 1.32 26.3 1.79 34.2 表 5 聚类分析组分水化学数据统计结果
Table 5 Statistics of hydrochemical data of clusters
分类 参数 t/℃ pH TDS Na+ K+ Ca2+ Mg2+ Li+ ${\mathrm{CO}}_3^{2-} $ ${\mathrm{HCO}}_3^{-} $ Cl− ${\mathrm{SO}}_4^{2-} $ F− SiO2 HBO2 As A A1 最大值 67 7.50 1.75 450.00 57.00 25.85 2.72 11.50 476.24 543.07 73.53 16.30 194.30 202.50 2.67 最小值 48 6.60 1.33 310.00 34.00 9.60 1.05 7.00 0 369.36 350.31 46.25 9.70 134.00 148.00 0.92 平均值 57 7.10 1.49 375.00 43.33 17.10 2.02 9.50 407.32 434.48 58.56 12.50 162.20 169.20 1.74 A2 最大值 90.5 9.00 1.89 467.50 60.50 13.80 5.23 13.15 142.23 439.00 623.65 90.87 14.90 247.60 303.70 4.00 最小值 80 7.70 1.60 400.00 34.00 0.20 0.15 9.10 0 127.89 468.00 8.42 6.20 124.80 203.00 1.50 平均值 84 8.49 1.72 433.71 51.14 5.68 2.80 11.76 51.49 259.33 525.38 51.30 12.07 175.19 264.70 2.51 最大值 91 9.70 2.65 637.25 131.00 72.00 14.59 28.60 209.07 1531.46 623.65 362.44 24.30 258.00 303.70 4.00 最小值 39 6.60 0.64 149.59 9.76 0.20 0.10 0.50 0 4.64 19.00 5.25 2.36 56.30 3.50 0 平均值 74 8.03 1.55 375.92 47.44 20.59 4.13 8.29 35.31 439.82 337.20 92.05 10.62 144.11 147.80 1.35 B 最大值 94 9.14 4.40 1253.60 138.28 48.12 13.62 24.92 261.65 1513.46 896.96 389.7 15.27 240.17 168.12 1.47 最小值 44 7.01 3.54 831.30 105.80 0 0 16.71 0 969.56 627.52 125.31 7.20 131.61 59.23 0.58 平均值 74 8.09 3.99 1062.57 126.09 9.85 2.92 21.99 68.11 1329.68 815.74 244.51 12.71 160.33 138.00 1.23 C 最大值 51 8.00 2.56 570.00 72.50 117.09 28.10 0.61 1020.00 38.20 798.00 6.65 86.86 20.91 最小值 29 6.80 0.67 150.00 8.20 48.00 10.21 0.10 0 482.00 14.30 132.00 2.06 31.10 11.00 0 平均值 43 7.32 1.82 420.49 49.70 94.03 19.00 0.44 833.31 29.55 536.11 4.67 65.62 15.64 表 6 因子分析中各物理化学指标因子载荷
Table 6 Factor loadings of various physicochemical indices derived from factor analysis
指标 1 2 3 旋转前F1 旋转后F'1 旋转前F2 旋转后F'2 旋转前F3 旋转后F'3 t 0.691 0.055 −0.344 0.711 −0.107 0.313 pH 0.691 −0.023 −0.456 0.868 −0.308 0.163 TDS 0.583 0.986 0.801 0.077 −0.065 0.083 Na+ 0.650 0.970 0.738 0.178 −0.112 0.088 K+ 0.585 0.932 0.748 0.053 0.032 0.176 Ca2+ −0.731 0.009 0.439 −0.781 0.100 −0.356 Mg2+ −0.658 −0.016 0.379 −0.750 0.195 −0.228 ${\mathrm{CO}}_3^{2-} $ 0.669 0.199 −0.183 0.702 −0.265 0.138 ${\mathrm{HCO}}_3^{-} $ 0.056 0.857 0.934 −0.276 −0.234 −0.344 Cl− 0.846 0.742 0.403 0.291 0.246 0.551 F− 0.607 0.041 −0.352 0.842 −0.468 −0.035 HBO2 0.648 0.069 −0.215 0.217 0.673 0.931 As 0.738 0.101 −0.242 0.356 0.555 0.880 -
[1] CAN M F,BAŞARAN C,YILDIZ A,et al. Lithium extraction from geothermal waters;a case study of Ömer–Gecek (Afyonkarahisar) geothermal area[J]. Turkish Journal of Earth Sciences,2021,30(9):1208−1220.
[2] ZANTE G,BOLTOEVA M,MASMOUDI A,et al. Lithium extraction from complex aqueous solutions using supported ionic liquid membranes[J]. Journal of Membrane Science,2019,580:62−76. DOI: 10.1016/j.memsci.2019.03.013
[3] SWAIN B. Recovery and recycling of lithium:A review[J]. Separation and Purification Technology,2017,172:388−403. DOI: 10.1016/j.seppur.2016.08.031
[4] WANG Hongshuang,CUI Jinjie,LI Mingli,et al. Selective recovery of lithium from geothermal water by EGDE cross–linked spherical CTS/LMO[J]. Chemical Engineering Journal,2020,389:124410. DOI: 10.1016/j.cej.2020.124410
[5] HE Maoyong,LUO Chongguang,YANG Hongjun,et al. Sources and a proposal for comprehensive exploitation of lithium brine deposits in the Qaidam Basin on the northern Tibetan Plateau,China:Evidence from Li isotopes[J]. Ore Geology Reviews,2020,117:103277. DOI: 10.1016/j.oregeorev.2019.103277
[6] 赵元艺. 中国盐湖锂资源及其开发进程[J]. 矿床地质,2003,22(1):99−106. DOI: 10.3969/j.issn.0258-7106.2003.01.012 ZHAO Yuanyi. Saline lake lithium resources of China and its exploitation[J]. Mineral Deposits,2003,22(1):99−106. DOI: 10.3969/j.issn.0258-7106.2003.01.012
[7] GUO Qinghai,LIU Mingliang,LI Jiexiang,et al. Fluid geochemical constraints on the heat source and reservoir temperature of the Banglazhang hydrothermal system,Yunnan–Tibet geothermal Province,China[J]. Journal of Geochemical Exploration,2017,172:109−119. DOI: 10.1016/j.gexplo.2016.10.012
[8] 多吉. 典型高温地热系统—羊八井热田基本物征[J]. 石油知识,2002(4):4−6. DUO Ji. Typical high temperature geothermal system-basic features of Yangbajing geothermal field[J]. Petroleum knowledge,2002(4):4−6.
[9] 侯增谦,李振清,曲晓明,等. 0.5 Ma以来的青藏高原隆升过程:来自冈底斯带热水活动的证据[J]. 中国科学(D 辑):地球科学,2001,31(增刊1):27–33. HOU Zengqian,LI Zhenqing,QU Xiaoming,et al. The uplifting processes of the Tibetan Plateau since 0.5 Ma BP:Evidence from hydrothermal activity in the Gangdise Belt[J]. Science in China Series D:Earth Sciences,2001,31(Sup.1):27–33.
[10] 魏帅超,张薇,付勇,等. 我国地热水中锂元素分布特征及资源开发利用[J/OL]. 中国地质,2024:1–32 [2024-01-31]. http://kns.cnki.net/kcms/detail/11.1167.P.20230505.1132.012.html. WEI Shuaichao,ZHANG Wei,FU Yong,et al. Distribution characteristics and resource potential evaluation of lithium in geothermal water in China[J/OL]. Geology in China,2024:1–32 [2024-01-31]. http://kns.cnki.net/kcms/detail/11.1167.P.20230505.1132.012.html.
[11] 王晨光,郑绵平,张雪飞,等. 青藏高原南部地热型锂资源[J]. 科技导报,2020,38(15):24−36. DOI: 10.3981/j.issn.1000-7857.2020.15.003 WANG Chenguang,ZHENG Mianping,ZHANG Xuefei,et al. Geothermal–type lithium resources in southern Tibetan Plateau[J]. Science & Technology Review,2020,38(15):24−36. DOI: 10.3981/j.issn.1000-7857.2020.15.003
[12] 王登红,代鸿章,刘善宝,等. 中国锂矿十年来勘查实践和理论研究的十个方面新进展新趋势[J]. 地质力学学报,2022,28(5):743−764. DOI: 10.12090/j.issn.1006-6616.20222811 WANG Denghong,DAI Hongzhang,LIU Shanbao,et al. New progress and trend in ten aspects of lithium exploration practice and theoretical research in China in the past decade[J]. Journal of Geomechanics,2022,28(5):743−764. DOI: 10.12090/j.issn.1006-6616.20222811
[13] GUPTA H K,ROY S. Geothermal energy:An alternative resource for the 21st century[M]. Amsterdam:Elsevier,2007.
[14] 赵平,多吉,谢鄂军,等. 中国典型高温热田热水的锶同位素研究[J]. 岩石学报,2003,19(3):569−576. DOI: 10.3969/j.issn.1000-0569.2003.03.023 ZHAO Ping,DUO Ji,XIE Ejun,et al. Strontium isotope data for thermal waters in selected high–temperature geothermal fields,China[J]. Acta Petrologica Sinica,2003,19(3):569−576. DOI: 10.3969/j.issn.1000-0569.2003.03.023
[15] 张煜道,谭红兵,丛培鑫,等. 西藏羊八井–当雄断裂带地热系统B、Li、Rb、Cs富集机制[J/OL]. 沉积学报,2024:1–18 [2024-01-31]. https://doi.org/10.14027/j.issn.1000–0550.2022.129. ZHANG Yudao,TAN Hongbing,CONG Peixin,et al. Enrichment mechanism of B,Li,Rb,and Cs in the geothermal system of Yangbajing–Dangxiong rift,Tibet[J/OL]. Acta Sedimentologica Sinica,2024:1–18 [2024-01-31]. https://doi.org/10.14027/j.issn.1000–0550.2022.129.
[16] BIRKLE P,MERKEL B,PORTUGAL E,et al. The origin of reservoir fluids in the geothermal field of Los Azufres,Mexico:Isotopical and hydrological indications[J]. Applied Geochemistry,2001,16(14):1595−1610. DOI: 10.1016/S0883-2927(01)00031-2
[17] 刘爱利,王培法,丁园圆. 地统计学概论[M]. 北京:科学出版社,2012. [18] 郑新奇,吕利娜. 地统计学(空间统计分析)[M]. 北京:科学出版社,2018. [19] MOUKANA J A,ASAUE H,KOIKE K. Co–kriging for modeling shallow groundwater level changes in consideration of land use/land cover pattern[J]. Environmental Earth Sciences,2013,70(4):1495−1506. DOI: 10.1007/s12665-013-2235-0
[20] NIKROO L,KOMPANI–ZARE M,SEPASKHAH A R,et al. Groundwater depth and elevation interpolation by kriging methods in Mohr Basin of Fars province in Iran[J]. Environmental Monitoring and Assessment,2010,166(1/2/3/4):387−407. DOI: 10.1007/s10661-009-1010-x
[21] KUMAR V. Kriging of groundwater levels:A case study[J]. Journal of Spatial Hydrology,2006,6(1):80−92.
[22] SHAHROKHNIA M A,SEPASKHAH A R,JAVAN M. Estimation of hydraulic parameters for Karoon river by cokriging and residual kriging[J]. Iranian Journal of Science and Technology,2004,28(B1):153−163.
[23] SCHMIDT N. Genesis and distribution of lithium enriched pore brines at the Salar de Uyuni,Bolivia[J]. Freiberg Online Geoscience,2020,57:1−156.
[24] LE N D,ZIDEK J V. Statistical analysis of environmental space–time processes[M]. New York:Springer Verlag,2006.
[25] ARMIJO R,TAPPONIER P,MERCIER J L,et al. Quaternary extension in southern Tibet:Field observations and tectonic implications[J]. Journal of Geophysical Research:Solid Earth,1986,91(B14):13803−13872. DOI: 10.1029/JB091iB14p13803
[26] 吴中海,叶培盛,王成敏,等. 藏南安岗地堑的史前大地震遗迹、年龄及其地质意义[J]. 地球科学(中国地质大学学报),2015,40(10):1621−1642. DOI: 10.3799/dqkx.2015.147 WU Zhonghai,YE Peisheng,WANG Chengmin,et al. The relics,ages and significance of prehistoric large earthquakes in the Angang Graben in south Tibet[J]. Earth Science (Journal of China University of Geosciences),2015,40(10):1621−1642. DOI: 10.3799/dqkx.2015.147
[27] HA Guanghao,WU Zhonghai,LIU Feng. Late Quaternary vertical slip rates along the southern Yadong–Gulu rift,southern Tibetan Plateau[J]. Tectonophysics,2019,755:75−90. DOI: 10.1016/j.tecto.2019.02.014
[28] 孙红丽,马峰,刘昭,等. 西藏高温地热显示区氟分布及富集特征[J]. 中国环境科学,2015,35(1):251−259. SUN Hongli,MA Feng,LIU Zhao,et al. The distribution and enrichment characteristics of fluoride in geothermal active area in Tibet[J]. China Environmental Science,2015,35(1):251−259.
[29] 吴珍汉,胡道功,刘崎胜,等. 西藏当雄地区构造地貌及形成演化过程[J]. 地球学报,2002,23(5):423−428. DOI: 10.3321/j.issn:1006-3021.2002.05.006 WU Zhenhan,HU Daogong,LIU Qisheng,et al. The formation and evolution of tectonic landform of Damxung area in central Tibetan Plateau[J]. Acta Geoscientia Sinica,2002,23(5):423−428. DOI: 10.3321/j.issn:1006-3021.2002.05.006
[30] 吴珍汉,胡道功,吴中海,等. 西藏羊八井–当雄–谷露地堑的地质特征与形成时代[C]//青藏高原地质过程与环境灾害效应文集. 北京:地震出版社,2005:228−234. [31] 高洪雷,胡志华,万汉平,等. 西藏谷露地热田地热地质特征[J]. 地球科学,2023,48(3):1014−1029. GAO Honglei,HU Zhihua,WAN Hanping,et al. Characteristics of geothermal geology of the Gulu geothermal field in Tibet[J]. Earth Science,2023,48(3):1014−1029.
[32] 赵文津. 喜马拉雅山及雅鲁藏布江缝合带深部结构与构造研究[M]. 北京:地质出版社,2001. [33] 赵文津,赵逊,史大年,等. 喜马拉雅和青藏高原深剖面 (INDEPTH) 研究进展[J]. 地质通报,2002,21(11):691−700. DOI: 10.3969/j.issn.1671-2552.2002.11.001 ZHAO Wenjin,ZHAO Xun,SHI Danian,et al. Progress in the study of deep (INDEPTH) profiles in the Himalayas and Qinghai–Tibet Plateau[J]. Geologcal Bulletin of China,2002,21(11):691−700. DOI: 10.3969/j.issn.1671-2552.2002.11.001
[34] BROWN L D,ZHAO W,NELSON K D,et al. Bright spots,structure,and magmatism in southern Tibet from INDEPTH seismic reflection profiling[J]. Science,1996,274(5293):1688−1690. DOI: 10.1126/science.274.5293.1688
[35] 张萌,蔺文静,刘昭,等. 西藏谷露高温地热系统水文地球化学特征及成因模式[J]. 成都理工大学学报(自然科学版),2014,41(3):382−392. ZHANG Meng,LIN Wenjing,LIU Zhao,et al. Hydrogeochemical characteristics and genetic model of Gulu high–temperature geothermal system in Tibet,China[J]. Journal of Chengdu University of Technology (Science & Technology Edition),2014,41(3):382−392.
[36] 佟伟,廖志杰,刘时彬. 西藏温泉志[M]. 北京:科学出版社,1999. [37] 赵平,金建,张海政,等. 西藏羊八井地热田热水的化学组成[J]. 地质科学,1998,33(1):61−72. DOI: 10.3321/j.issn:0563-5020.1998.01.016 ZHAO Ping,JIN Jian,ZHANG Haizheng,et al. Chemical composition of thermal water in the Yangbajing geothermal field,Tibet[J]. Chinese Journal of Geology,1998,33(1):61−72. DOI: 10.3321/j.issn:0563-5020.1998.01.016
[38] 刘昭,蔺文静,张萌,等. 西藏尼木–那曲地热流体成因及幔源流体贡献[J]. 地学前缘,2014,21(6):356−371. LIU Zhao,LIN Wenjing,ZHANG Meng,et al. Geothermal fluid genesis and mantle fluids contributions in Nimu–Naqu Tibet[J]. Earth Science Frontiers,2014,21(6):356−371.
[39] 任加国,武倩倩. 水文地球化学基础[M]. 北京:地质出版社,2014. [40] 张洁,梁杏,刘延锋,等. 基于主成分的协克里金法对地下水砷空间分布预测[J]. 地球科学,2023,48(10):3820−3831. ZHANG Jie,LIANG Xing,LIU Yanfeng,et al. Co–Kriging method based on principal components to predict spatial distribution of arsenic in groundwater[J]. Earth Science,2023,48(10):3820−3831.
[41] 李卫东. 应用多元统计分析(第二版)[M]. 北京:北京大学出版社,2015. [42] GROENEVELD R A,MEEDEN G. Measuring skewness and kurtosis[J]. Journal of the Royal Statistical Society:Series D (The Statistician),1984,33(4):391−399.
[43] ZHANG Libo,CHAN L H,GIESKES J M. Lithium isotope geochemistry of pore waters from ocean drilling program Sites 918 and 919,Irminger Basin[J]. Geochimica et Cosmochimica Acta,1998,62(14):2437−2450. DOI: 10.1016/S0016-7037(98)00178-1
[44] 苏为华. 多指标综合评价理论与方法问题研究[D]. 厦门:厦门大学,2000. SU Weihua. Research on the theory and methods of multi indicator comprehensive evaluation[D]. Xiamen:Xiamen University,2000.
[45] 郑绵平,王秋霞,多吉. 水热成矿新类型:西藏铯硅华矿床[M]. 北京:地质出版社,1995.







 下载:
下载: